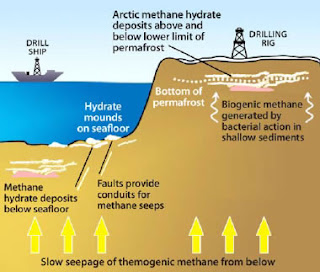
From the first article, we know that methane gas is released from the seabed and increases the concentration of methane during the summer months. However, there is very little movement in the Arctic Ocean during the summer. An important term to introduce here is stratification. Stratification is a process where the layers and bodies of the ocean mix ingredients. In the picture below, fluids of varying densities will layer themselves in a column. We've seen this in elementary science class.
If we take a stirring rod and attempt to mix these fluids, we may find that some mix better than others. We know that oil in water won't mix but will break up into bubbles of oil until we stop stirring. During the summer months, the Arctic Ocean is density layered and very little gas exchange occurs. Methane may be released but it will not enter the atmosphere until the conditions change and the waters begin mixing again through temperature (thermocline), density (pycnocline), oxygenation (chemocline), or salinity (halocline) variations brought on by the changing of seasons or weather events.
About a week later, the second article was released and it sounded a bit ominous. However, we are finding out how important these hydrothermal vents are to the biosphere of the ocean. We do know that methane is 25% more potent as a greenhouse gas than carbon dioxide. That is why scientists and ecologists have been concerned about methane releases into the atmosphere.
 |
| carbon dioxide |
 |
| methane |
Then a fascinating result arose from studying the ecosystem of the methane seeps. The life forms that surround these vents consume a whopping 90% of the methane released! But there is a problem. These vents release more than methane and are being considered by mining companies as possible drill sites for copper, zinc, gold, lead, and silver. Drilling would destroy these habitats as we are beginning to learn how much methane they are withholding from the atmosphere.
 |
| Source: geology.com |


